| CARVIEW |
Do you fear clusters of small holes, or irregular patterns? If so, chances are you might have trypophobia. Trypophobia is a phobia that manifests when exposed to images or objects that have groupings of perforations or holes on the surface. An example of an object that elicits a response characteristic of trypophobic is a lotus seed-head. Trypophobia is thought to be an evolutionary advantage and has the same neural response to that associated with snakes and other harmful and deadly organisms that display similar patterning.
You may be asking yourself how trypophobia can be related to anything other than utter repulsion and disgust, especially anything inside of the human body. Well, I have news for you! In the following paragraphs, I will shed light on a biological process that may induce some trypophobic responses. So, now that I have succeeded in hopefully grabbing your attention, allow me to introduce the Complement System.

CC-BY-SA-3.0 Credit: KENPEI
What is the Complement System, and Why does it Matter?
Let me begin with some context regarding what exactly the Complement System is, and how it functions within the immune system. In the human body, there are copious proteins that have antimicrobial properties, and are primarily found circulating in the blood. The proteins that are pertinent to this discussion consist of approximately thirty and are collectively called the Complement System. This system belongs to the innate immune system, and functions to complement the acquired immune system, hence the name. The ability of the innate immune system to engage and function alongside the acquired immune system is biologically fundamental to the destruction and protection against pathogens. As stated, the Complement System is comprised of approximately thirty proteins, which are continually produced by the liver, and interact to elicit an immune response. The interaction of complement proteins is dependent upon activation, which results from the interaction between complement protein and pathogen antibody. Complement proteins are classified as pro-proteins, meaning that they are only activated after a portion of the protein is cleaved.
Functioning of the Complement
Now that you are armed with the basics of the Complement System and its involvement with the acquired immune system, how about we start to fill your toolbox with more detailed functions of the Complement System? Before we commence, I must confess-I may have lied for simplicity sake. As I will describe, the Complement System is activated multiple ways, and not just through antibody interaction. So, with that out of the way, allow me to continue. The early components of the Complement System consist of three categories of proteins, with each belonging to three separate and unique pathways of complement activation. These pathways include: the classical pathway, lectin pathway, and the alternative pathway.
These pathways function to activate the Complement System to trigger phagocytosis, inflammation, or a membrane attack to destroy a pathogen. All the three pathways result in the production of a protease (protease C3-convertase) that functions in the potential opsonization of the pathogen. The classical pathway is activated through the binding of the complement protein C1 to the antibodies IgG and IgM on pathogen surface, which allows for recognition of the pathogen by the innate immune system. The adaptive immune system produces antibodies that recognize and bind C1 to results in phagocytosis of the pathogen. The mannose-binding lectin pathway is similar to the classical pathway, but differs in terms of the binding target. For this pathway, mannose-binding lectin binds to mannose, glucose, or other sugars on the surface of the pathogen’s membrane. This binding allows for the recruitment of the adaptive immune system to destroy the pathogen.
Finally, the alternative pathway is primarily responsible for opsonization of the pathogen through the formation of the membrane attack complex (MAC). The formation of the MAC occurs through the binding of multiple C proteins to the membrane of the pathogen. These proteins function to form a pore through the lipid bilayer of the pathogen, which results in the influx of water in to the cell.
]]>Ever been jealous of a fish? Well, you’re about to be.
The Mexican cavefish, Astyanax mexicanus, only needs two hours of sleep in a 24 hour cycle. It also doesn’t face any adverse effects developmentally or on its health even when it’s deprived of sleep. Sleep patterns vary immensely among all the organisms in the world; why did us humans have to get stuck with requiring a solid eight hours? So not fair. Honestly though, is it just me or do you also not feel recharged even after a solid eight hours of sleep? I can’t remember the last time I didn’t feel tired…I think the last time I got a full night of sleep was in the womb. On the bright side, at least we don’t need as much sleep as a brown bat does- one of those guys’ needs about 20 hours of sleep a day…but then again, those guys can fly, so never-mind, they still get the better end of the stick.
Cavefish are model organisms, along with Drosophila melanogaster and zebrafish, that are used to help scientists understand sleep and wakefulness better, along with why sleep cycles vary so much among organisms. For example, the circadian clock network has been studied extensively in Drosophila.
There are two different populations of A. mexicanus. One is a surface population, which inhabits rivers, and there are also multiple populations that inhabit caves. The surface population doesn’t have the same sleep pattern as the one that resides in caves. They’re lame like us and require eight hours of sleep. Cavefish have bodies that contain very little pigment, and they are also blind. Both of these traits help them regulate their sleep.
A journal article published on A. mexicanus this year reported that signalling of the HCRT gene which encodes the neuropeptide hypocretin or orexin plays an important role in sleep regulation. HCRT inhibition in cavefish was seen to increase their requirement for sleep. This finding may possibly lead scientists to finding a way for humans to require less sleep, via enhanced HCRT signalling.
There were many findings as to how the underlying mechanisms of sleep are controlled in cavefish, in relation to the HCRT gene. One finding showed that the HCRT neurons are regulated by leptin, which is an adipose peptide hormone. Surface populations of cavefish have lower adipose levels, which would explain the difference between the populations: starvation lowers leptin levels, which in turn inhibits HCRT expression to promote sleep in cavefish.
And you thought that you were always sleepy: Narcolepsy
The neuropeptide hypocretin is found in the hypothalamus of many organisms, including humans. Deficiencies in HCRT include sleep disorders such as narcolepsy in humans. Narcolepsy is characterized by excessive sleepiness, sleep paralysis, hallucinations and can also be associated with cataplexy. People with narcolepsy can fall asleep involuntarily throughout the day during normal activities. HCRT neurodegeneration is thought to be associated to autoimmune processes. Narcolepsy with HCRT deficiency is linked to human leukocyte antigen (HLA) and T-cell receptor (TCR) polymorphisms. This suggests that an autoimmune process targets a single peptide that is specific to HCRT cells via HLA-peptide-TCR interactions. Processes such as molecular mimicry and bystander activation have also shown association to the disorder because of disease onset in children and associations with Streptococcus pyogenes and H1N1 infection and vaccination. Molecular mimicry is a mechanism of autoimmune disease. It’s when a foreign antigen has similarities with self-antigens. Self-antigens are the antigens on your own cells. This mechanism can lead to autoimmune diseases because the immune system may recognize self-antigens as foreign and this can lead to the destruction of specific tissues and organs. Bystander activation is when T-cells are activated without T-cell receptor stimulation. In this case, T-cells end up skipping over particular immune regulatory checkpoints.
Current treatments for narcolepsy are symptomatic and they consist of therapeutics that promote wakefulness. These therapeutics raise presynaptic dopamine release and anti-cataplectic agents that activate monoaminergic neurotransmission. Research is still being done towards developing hypocretin replacement therapy.
Sleep serves a major role in biology, and model organisms have assisted in understanding the mechanisms of sleep, and in particular, the neurobiology of the HCRT system, but it is still a huge mystery.
In the meanwhile, who wants to sign a petition for Apple to come out with a charger for humans? I mean, since it can’t seem to make efficient chargers for iPhones anyways, it might as well try something different.
]]>

In recent years, there has been a marked increase in autoimmune diseases such as Type I Diabetes (T1D), asthma, and allergies. Surprisingly, cases of an increase in autoimmune diseases and allergies were more pronounced in developed countries which also reported a decrease in common infection cases as well as better disease control. Originally, it was proposed that these changes were due to genetic factors however, this was highly unlikely given the short period in which the rise was first observed. Upon further analysis, it was suggested that the environmental conditions in which people lived in developed countries were playing a large role in this effect. The commonality amongst all developed countries where this was the case had to do with hygienic measures put in place, such as with the decontamination of water supply, pasteurization and sterilization of milk and other food products, vaccinations against common childhood infections, and the wide use of antibiotics to combat illnesses caused by bacteria. This begs the question of what the correlation between disease control and the production of autoimmune diseases are and why such trend are commonly observed. The answer to this question was first proposed by a hypothesis called the Hygiene Hypothesis.
Before we get into the hypothesis, let’s take a quick detour and talk about the human gut (I know it may seem random, but trust me when I say there’s a method to the madness).
Diversity of gut flora. Image Credits: OpenClipart-Vectors Creative Commons Zero [Public Use].
The human body is home to a largely diverse population of microorganisms. In fact, there are 10-fold more microorganisms present in the body that there are human cells. Combined, these microorganisms contain 150-fold more genes than the human body. The role of these microorganisms are predominantly with the development of immune responses, digestion of molecules, production of vitamins, and detoxification of harmful chemicals. They are largely found on the surface of the skin, in the nose, mouth, and gut, and all play specific and unique roles, aiding in the sustenance of the human body. Microbes found in the gut are responsible for the development of the immune system and undue changes in the microbiota can cause a wide range of diseases such as irritable bowel syndrome, autoimmune diseases and allergies, obesity and metabolic disorders, and neuropsychiatric disorders such as autism and depression. The development of gut flora is initiated immediately after birth and is enhanced throughout the life of the person. However, the most important period of this development is within the first 6 months of life. To learn more about the gut flora, check out this link.
Now that I’ve given you this seemingly useless information about your gut flora, let’s talk about how it relates to the hygiene hypothesis.

Teddy bear with flu vaccination. Image Credits: PxHere Creative Commons Zero [Public Use]
The theoretical solution to this would be for children in their early stages to be exposed to as much environmental factors to help with the development and diversification of the gut and intestinal flora. However, having a doctor prescribe a baby to roll around in mud 2-3 times daily and sneeze on other children for the first 6 months of life would be hardly taken well by parents. So, the question now becomes, how can one aid in the development of an infant’s microbiota without causing adverse dangers such as contracting deadly infections or putting the child in harm? Many have suggested introducing the use of probiotic supplements, but possible side effects can arise from this for example, the change in composition of the flora in such a way that mutants arise. In a way, probiotic supplements are just another way of taking antibiotics to help solve the problem but is it really solving the problem?
Am I saying don’t wash your hands? Definitely not. I’m just saying that maybe the next time your sibling sneezes on you, it might not be a completely bad thing. In fact, consider thanking them… Again, I’m kidding.
If you managed to make it to the end, first, I #congratulate you (  ), and second, consider checking out this revision of the hygiene hypothesis and a more in-depth discussion on the role of endotoxins and Th2 in the development of autoimmune diseases and allergens.
), and second, consider checking out this revision of the hygiene hypothesis and a more in-depth discussion on the role of endotoxins and Th2 in the development of autoimmune diseases and allergens.

By Ferran Pestaña from Barcelona, España (Abeja – Anthophora plumipes 03) [CC BY-SA 2.0 (https://creativecommons.org/licenses/by-sa/2.0)], via Wikimedia Commons
That’s why the phenomena known as Colony Collapse Disorder (CCD) is so scary. This occurs when the majority of bees in a population disappear from the hives all at once, typically while out foraging, leaving behind a queen bee, some nurse bees, and a whole lot of food stores. Without the mature worker bees around to deliver pollen and nectar back to the hive, the hive collapses. Now dead bees don’t necessarily mean CCD, but the absence of any bodies rules out things like acute pesticide poisoning . CCD was first identified in 2006, and has been the center of many investigations since then. One study identified 61 possible factors contributing to CCD, but couldn’t pinpoint the cause to any single one. However, one seemingly main culprit was highlighted: the transmittance of viruses to bees by parasites, namely the varroa mites.

By CheepShot (Exposed Bee Hive) [CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons
There are several vector-transmitted viruses that have been studied, including deformed wing virus (DWV), black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), acute bee paralysis virus (ABPV), and kashmir bee virus (KBV). While all these viruses present differently and have different symptoms, nearly all are fatal. Typically, these viruses attack the bees through oral ingestion of infected food. They travel along the bee’s digestive system to the gut, where they must get past the epithelial cells. These cells are constantly being replaced and are protecting by membranes and filters, making it quite a process for the virus to actually infect the organism. Varroa mites make it possible for the virus to bypass this defense mechanism entirely.

By Alias 0591 from the Netherlands (Humblebee) [CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons
Humans live in a co-evolutionary association with these commensal microorganisms which are present both internally (e.g gut) and externally. The gut microbiome plays part in directing and facilitating developmental processes in the brain with long term implications to health.
The gut microbiome diversity: Activity and effects.
Dysbiosis – a state of imbalance between the good and bad bacteria – is fundamental to understanding the effects our microbiome flora has on mental and general health. Perhaps the area of highest importance is understanding the diversity of microbiomes present in the gut, and in what relative amounts to each other do they need to be present in. Studies have shown that the activity of one microbe in the gut can influence the activity of another, the absence of a microbe can also be quite detrimental. The microbiome present in our gut have various important roles, for instance, they help synthesize vitamins and co-factors that we lack, a decrease in the number of microbes present in the gut also results in a decrease in short-chain fatty acids which is important for neurological function. Numerous studies conducted have all shown similar results with little to no difference on the effect of dysbiosis. These effects are seen to be potential precursors to mental health disorders like Autistic spectrum disorder (ASD) and Schizophrenia , the gut microbiome has also shown to influence fear and anxiety. However, there is no substantial evidence that the gut microbiome influences Schizophrenia.
What studies have shown in Autism.
Studies have shown that our microbiome can modulate gastrointestinal physiology, immune function and behavior. The results of dysbiosis was shown using mice models. In an experiment carried out by a group of researchers, two groups of mice were used, mice delivered in a germ-free environment (by c-section), and mice delivered through the maternal vaginal canal. The vaginal canal is the first source of microbes we come in contact with when delivered. It was observed that the mice delivered in germ-free environment showed autistic behaviors. They expressed decreased sociability, while mice delivered naturally showed no sign of decreased sociability. However, if applied to humans, one thing is left out; children delivered through the vaginal canal that still develop autism and those given birth to through a c-section that do not develop it. Nevertheless, there are multiple mechanisms that come in play with the development of autism besides dysbiosis.
These germ-free mice also had an exaggerated hypothalamic-pituitary axis with elevated corticosterone and adrenocotrophic hormone, both of which are present in stressful situations. This suggested that the microbiome present in our gut may determine how we are able to handle stressful situations. Germ-free mice showed abnormalities in brain gene-expression and neurophysiology, reiterating the effects on the nervous system.
How does this happen?
It is unclear how exactly the brain and the gut microbiome communicate, it seems nearly impossible that the simple bacteria’s in our gut can interact with such a complex organ, the brain. It is astonishing to think they can. Even more shocking is that they can function without the brain, and are in fact termed as our second brain. It is suggested that the brain and the gut microbiome are linked and communicate through the vagus nerve. Our brain is able to process information and send it to the gut microbiome, and the microbiome is also able to detect external stimuli and send to the brain. There seems to be a bidirectional communication between the gut microbiome and the nervous system through the vagus nerve and humoral component, including the immune system and the hypothalamus-pituitary adrenal axis. Our immune system is also at a loss in the event of a dysbiosis. Specific bacteria’s are required for proper immune function. Most individuals with a dysbiosis have a compromised immune system.
Reversing the effect of dysbiosis?!!!!
Following the observed behavioral difference between the germ and germ-free mice, the researches treated the germ-free mice with Lactobacillus Reuteri. This treatment reversed the decreased sociability in the germ-free mice, the mice started interacting more with other mice afterwards. This suggests the germ-free mice lacked Lactobacillus Reuteri, which may be the key bacteria in play with social behaviors. In individuals with a ASD, it has also been found that there is a high amount of Clostridium, however, its dominance does not fully explain its purpose.
Furthermore, shocking discoveries have been made in mice treated with bacteria Toxoplasma Gondii . These mice were attracted to cats instead of being afraid of them. This suggests that fear and anxiety can be treated through manipulating our gut microbiome.
The future of this discovery.
The discovery on the effects of our microbiome community is a big feat in the medical field. However, there is much work to be done on understanding the mechanism by which these gut microbes influence mental health. It does not clearly show whether the presence of specific microbes are a cause or a side effect to these mental health conditions. Nevertheless, if found to be causative, it is a discovery that would positively affect the lives of millions around the world. The future outcomes of this medical research is bright and assuring.
]]>Our modern world is filled with mad and anxious people. According to Statistics Canada, 23.0% of Canadians aged 15 and older (6.7 million people) reported that most days were ‘quite a bit’ or ‘extremely stressful’ and about 1 in 5 Canadians will experience mental illness in some form. My fellow science colleagues can surely attest to this.
The balancing act of juggling a full course load and the pressures of high performance, working part-time jobs, participating in extracurricular activities, (the list goes on) leaves little time to do anything else – like sleeping – even though sleeping less than 5 hours a day has been shown to decrease immune function, increase the build-up of beta amyloids in the brain, reduce cognitive function and memory, and a multitude of other effects.

Is there any weed for a weed like me? I’m stressed….  (Photo by user FoeNyx under the GNU Free Documentation License)
(Photo by user FoeNyx under the GNU Free Documentation License)
Students or even humans aren’t the only ones however. Plants, microbes and fungi experience stress too, but for us, that’s a good thing. When their innate immune response is triggered by stressors such as toxins or pollutants, secondary metabolites are produced which are essential to their adaptive defense mechanisms in changing environments – a phenomenon known as hormesis. These secondary metabolites can be harnessed by humans for their immuno-stimulatory or anti-inflammatory effects, a property which is currently being investigated by some of your very own profs at UNBC!
But, as always, there is a caveat. Stress is what keeps us alive. It increases our arousal and keeps us motivated to do something, anything – rather than getting bored. In fact, would you rather inflict pain on yourself rather than be bored? The answer is surprising. You probably would.
Aside from this, stress is what makes humans highly adaptable to dangerous and life-threatening situations. There is a famous story of a sole plane crash survivor named Juliane Koepcke who lived through 10 days in the forest without food while sustaining several serious injuries, including an arm that was infested with maggots. When faced with danger or fear, our amydala, the “fear centre” of our brain is activated and adrenaline is released manifesting in “superhuman” powers which only arise during these “do or die” scenarios.
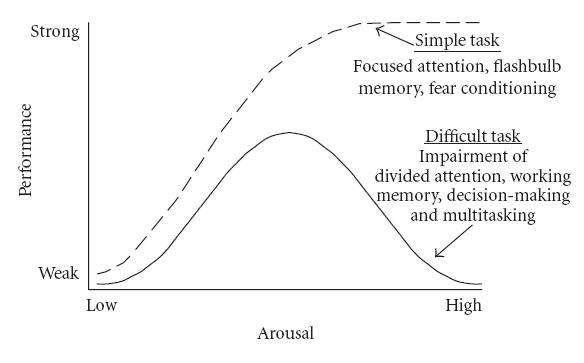
The Yerkes-Dodson Model (Image under Creative Commons CC0 1.0 Universal Public Domain Dedication originally from Yerkes and Dodson 1908)The psychological Yerkes Dodson model is a good indicator that shows to what extent stress can improve our performance and motivate us to achieve our goals, but what happens when we pass that threshold?
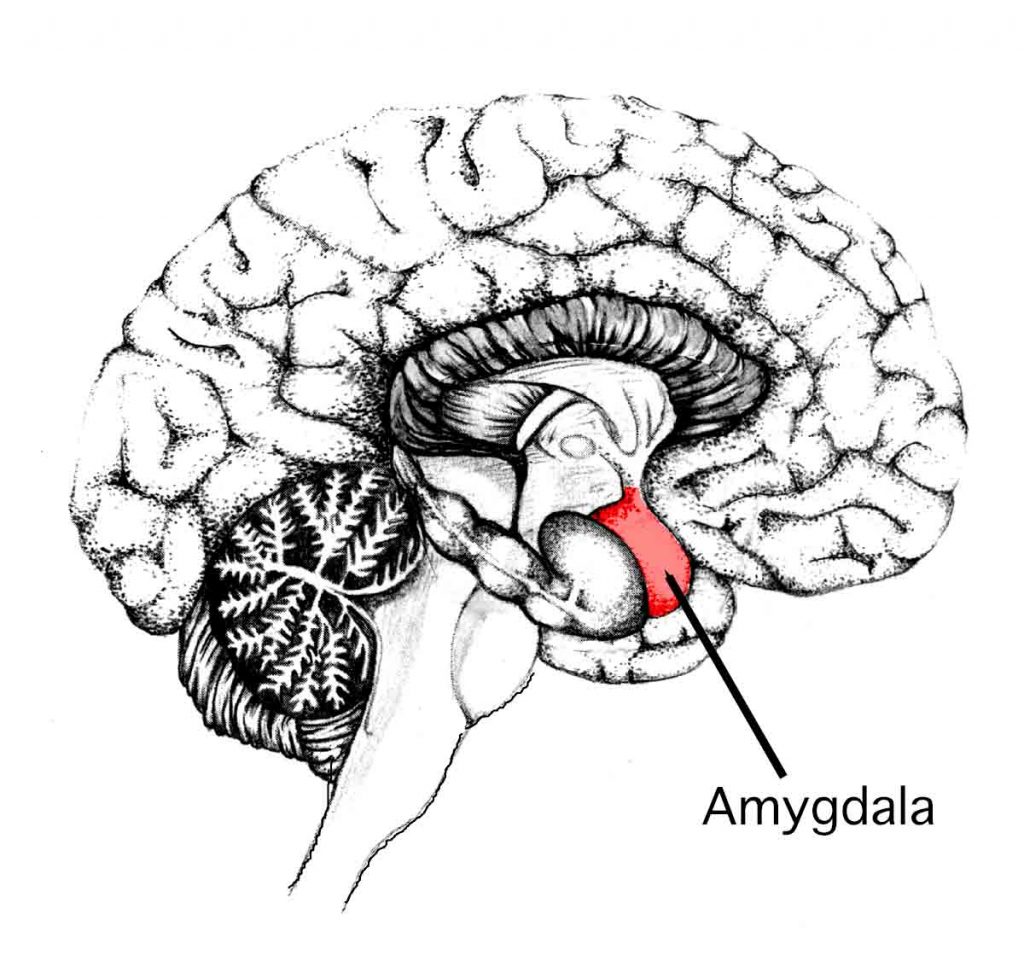
If you don’t have the amygdala, you won’t experience fear or stress… but it also means you’ll probably die. (Image under Creative Commons CC0 1.0 Universal Public Domain Dedication from user Sgerstenberg)
Stress has many physiological effects on the body; scraps from our primitive past when our primary concern was whether we could outrun a bear rather than worry about being late for work. More specifically, it activates the flight or flight response via our sympathetic system, releasing hormones such as cortisol and epinephrine that present numerous effects on the body. This is great in the short term. If chronic however, it can cause many harmful symptoms such as weight gain, hypertension, mental illness and yes, even death. In Japan, the prevalence of death from overworking has even led to the development of its own word – Karoshi.
The intersectionality between our mind and how it can influence our physiology is best encapsulated by the emerging field of psychoneuroimmunology. This discipline places emphasis on how our subjective behavioural experiences of stress can be extrapolated to a weakened immune system leading to tumorigenesis, disease, and can even affect cancer survivorship. In addition, it explains how those who experience mental health issues are much more likely to participate in health-harming activities such as smoking, drugs and alcohol consumption because they are less likely to “care”. Therefore, it has become important to recognize the holistic health of the individual rather than treating the patient by their physical symptoms alone. This is a key concept in the placebo effect, a powerful yet underestimated factor in our health.
However, stress is a very subjective experience. Not everyone thinks diving off a cliff is stressful or getting a 50% on a midterm is particularly life-threatening. For daredevils, people who thrive on perilous encounters, being in a constant state of stress is like a drug to them. Scientists who study these individuals have suggested both a genetic and environmental factor in how people perceive stressful situations. So, can we change the way we react to a stressor and can it help us with mental illness?
Historically, people who suffered from anxiety disorders and mental illness did not even see it as problem – a view which still persists in many developing countries. Though the stigma of mental illness has been lifted in our modern society, people are being diagnosed with mental illnesses more now than ever before, (some misdiagnosed) with the treatment in the form of prescription drugs to manage their problems – a typification shared with type II diabetes. Not only does this place a huge financial burden on the Canadian healthcare system, but it also does little to fix the real cause. Students shouldn’t be taking pills because they are depressed from having no life outside of school, but the school system should change so that students aren’t depressed. A big difference. Additionally, there are many other conducive ways of coping with stress such as exercise, meditation, or painting.

Edvard Munch might be crazy… but I least he’s crazy with style.(Photo under Creative Commons 2.0 Generic License)
If there is anything that I have learned from looking at “The Scream”, from an artist who in our time would be diagnosed with psychosis and bipolar disorder, it is that stress and succumbing to it doesn’t make us weak or feeble. If anything, Munch’s depiction of himself staring at us with his mouth agape from anguish and panic provides us with a glimpse of reality, humanity, and compassion. So this exam season, thank Stress for making you study, but if you need to… scream it out. It’s healthy.
]]>We begin our lives as little congregations of proliferating cells. We expand and grow, familiar shapes begin to form, segments become identifiable with functional specificity. Somewhere along, we become “life” although where along is arguable. Eventually we are large-headed tube feeders somersaulting and kicking in the maternal hotel where light is dark and all is warm and worry is a foreign characteristic for outsiders. How ironic that we both leave the womb and enter the tomb with a light at the end of a dark tunnel (unless of course you are born as the “cheeky” type and breech the status quo). Nevertheless, our little life-in-the-works has not acquired the complete “fit for the world” tool kit prior to parturition just yet; after all, “the night is dark and full of terrors” and we are all in need of a protector…or protectors…or 10-100 trillion protectors—yes that seems best. A push, a squeeze, maybe even a plunge, clamp or snip, and we’re off on the trajectory of life yet, without even knowing it, the humblest of protectors have already begun their new life’s work: managing ours.
Figure 1. A cartoon drawing depicting a feisty bacteria ready for a fight. The image is released free of copyrights under CCO Creative Commons. Attribution not required.
It may not be overtly apparent, but we all have microbes (bacteria, fungi, protozoans, archaea) living and dying on us at all times. Humans, among other earthly inhabitants, are ecosystems for microbial communities which gives the terms “microbiome” (the taxa association) and “microbiota” (the list of microbes and their genes) interesting implications—here we are living on, and thus effecting, the Earth and the communities that we share it with and all the while there are symbionts treating us in similar ways. Our unique and individual microbiomes influence, or are influenced by, a mind-boggling amount of processes such as inflammation, anxiety, circadian rhythms and obesity, cancer, alleviation of type 2 diabetes, craving for junk food… the list goes on.
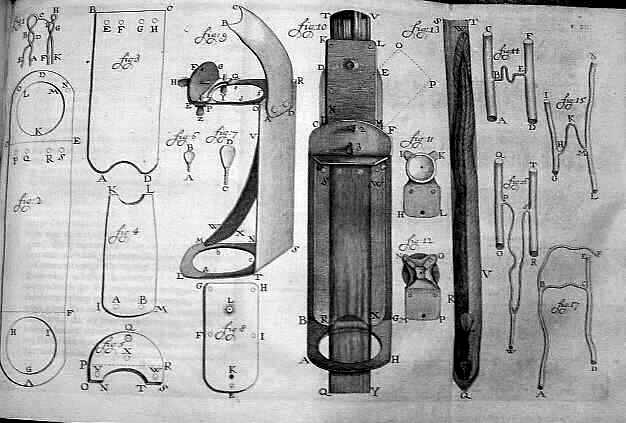
Figure x. A 1756 drawing of Antonie van Leeuwenhoek’s microscope he used in his research by Henry Baker. This image is released free of copyrights under the public domain of the United States. Attribution not required
It may come as no surprise that Robert Hooke and Antonie van Leeuwenhoek, pioneers of the early microscope, are attributed with the first description and discovery of a microorganism in 1665, the microfungus, Mucor. Sounding likely unintentionally adorable, Leeuwenhoek termed the first microbes “animalcules”. Now, with 353 years of advancement and metagenomic sequencing, our understanding is considerably more advanced. There’s an infinite set of questions here, but lets focus on where we actually acquire our micro-residents and some of the effects of acquisition circumstance.
The microbiome one obtains near or during birth may change over time with aging, stress, diet, environmental factors, etc. Focusing on where and when the microbiome develops, we will consider pre-birth (in utero), during birth (vaginal or cesarean), first foods, and environments. The idea that the microbiome is acquired during and after birth has been widely accepted for years which stems in reasoning from the “sterile womb paradigm” that has been considered dogma for over a century. Recently there have been several publications suggesting the existence of commensurable placental microbiomes that a fetus may obtain in utero. This was curious, but more so when discovering a critical assessment and study with more rigorous methodology essentially squashing the placental studies and attributing their results to i) their PCR and next-generation sequencing methodologies not having adequate detection limits for low density populations of bacteria, ii) contamination susceptibility of the methodologies and iii) environment (hospital), and finally, iv) wrongly interpreted stool samples findings—a fine example of the scientific method at play.
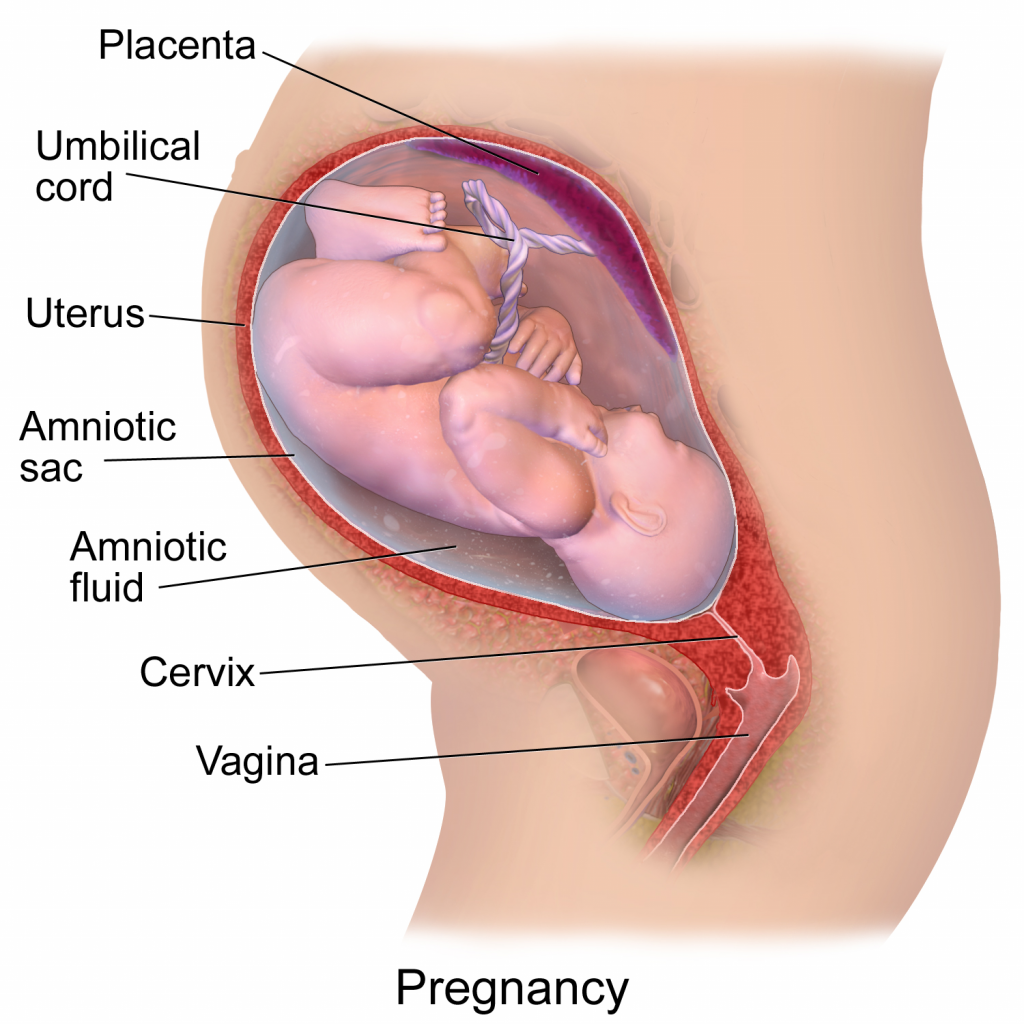
Figure 3. A diagram depicting a pregnancy well into gestational development. This image has been released free of copyright by Blausen.com staff (2014). Own work, CC BY 3.0
Without any intention to discourage necessary C-sections, there are considerable benefits to a vaginal birth, namely attributed to a vaginal microbiota coat applied during delivery. During pregnancy, a woman’s vaginal microbiome will undergo compositional changes by lowering diversity with dominance from spices of lactobacilli, clostridiales, bacteroidales, and actinomycetales. After vaginal delivery, a newborn’s microbiome will strongly reflect the microbiome of the mother’s vagina and feces whereas after cesarean delivery, it is more reflective of the mother’s skin and hospital environment. As we are all aware, hospital environments can be concerning to downright pathogenic at times which is why newborns can acquire pathogenic bacteria like Clostridium difficile, methicillin-resistant Staphylococcus aureus (MRSA), and pathogenic E. coli while lacking beneficial Bacteroidetes and Bi. Longum subsp. Infantis. Beneficial Bifidobacterium occasionally make their way onto newborns during a cesarean birth and when they do, pathogenic E. coli and C. difficile are not found; I think this is awesome, the microbial army your mother can give you will outcompete and destroy pathogenic marauders! For reasons relevant to the microbiome composition, a cesarean birth may increase risk of disease such as celiac disease, asthma, type 1 diabetes, and obesity. It sounds a bit inappropriate and awkward but, would rubbing a cesarean newborn on the mother’s vagina immediately after delivery be a terrible idea? —there’s a story for prom night!
As a newborn, your initial dining experiences play a considerable role in introducing new microbial communities. Breast feeding over formula has many benefits to an infant including the stimulation of neonatal microbiome maturation of the gut. Environments, such as hospital delivery rooms, can have lasting effects also; for instance, you may feel safe laying across your father’s perhaps burly and hairy chest as an infant, and you should because he just gave you another powerful microbial community of his own. Remember that maternal army of microbes that fends off bacterial marauders? The take away is that some species will stay for good, giving you a microbial signature that is unique and, in some cases, bacterial assemblages can be traced back to individual persons. So, what would happen if Neil DeGrasse Tyson, the astrophysicist, and Robert Krulwich from the Radiolab podcast shook hands; would their microbiomes mix, die off, or would they compete and declare a winner? I’ll leave you to find out.
Conclusion:
Whether you get your microbiome from your mother, your father, the foods you eat, the places you go, the people you meet or all the above, you have a microbiome. It influences your mood, your metabolism, your health, and is very much a part of you. Our world is infinitely complex and full of surprises, but I for one am grateful to know that a whole history of biological evolution is hanging out and navigating life with me.
]]>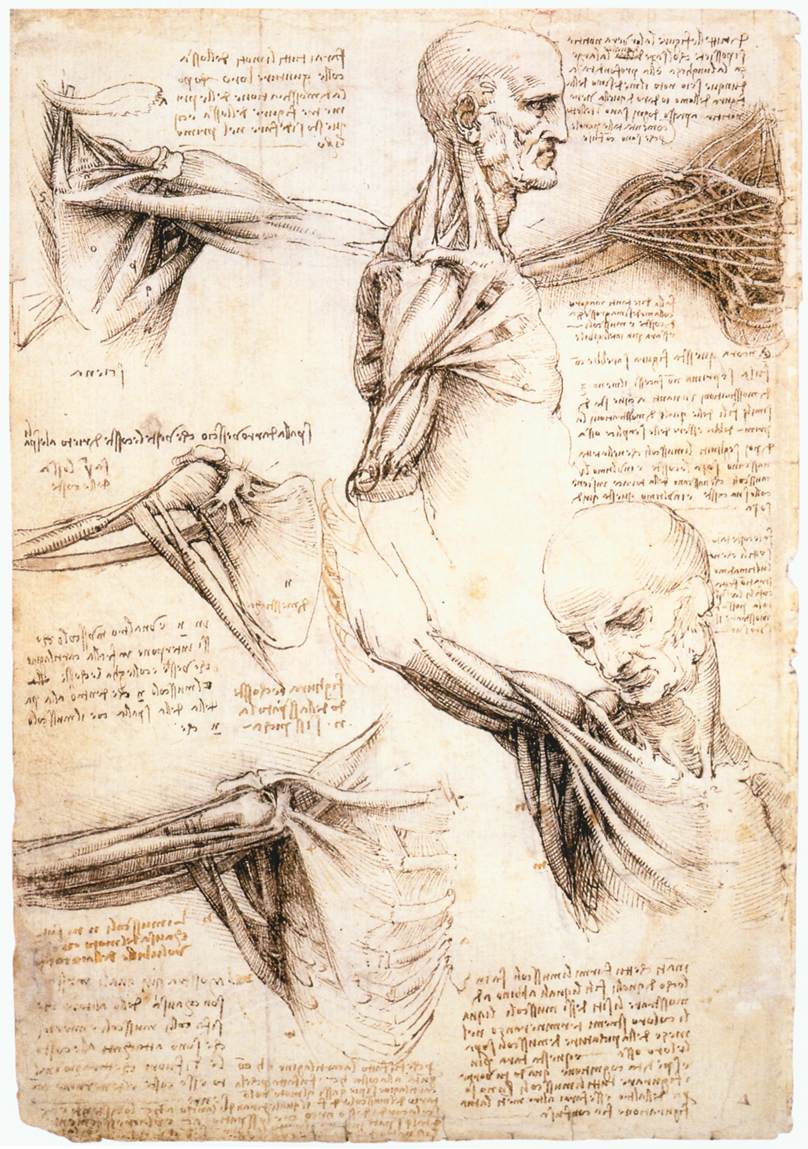
The muscles of the shoulder and arm, and the bones of the foot (c.1510–11) Author Leonardo da Vinci (PDM) Public Domain
In Dr. Payne’s “History of Medicine” lecture we discussed the early innovators of medical understanding and I must say I was slightly disappointed by the absence of Leonardo from that lecture. During the late 1480s, Leonardo began dissecting human and animal bodies to better understand the anatomy of life. Some of these sketches such as fetus in utero, and the heart and vascular system are the first on human record. His commitment to understanding the human body was clear on April 2nd, 1489, when he began his “Book entitled On the Human Figure.” Leonardo completed is first anatomy notebook known now as “Anatomical Manuscript B”. However, this book was mostly unused and empty as he had only access to one corpse at the time of the construction of the notebook. It was not until the year 1510 when Leonardo was able to partner with a young anatomy professor Marcantonio Della Torre, that he had access to cadavers necessary for his work. It was with this access Leonardo completed what is now known as “Anatomical Manuscript A” which consisted of 240 individual drawings over 13,000 words of notes.
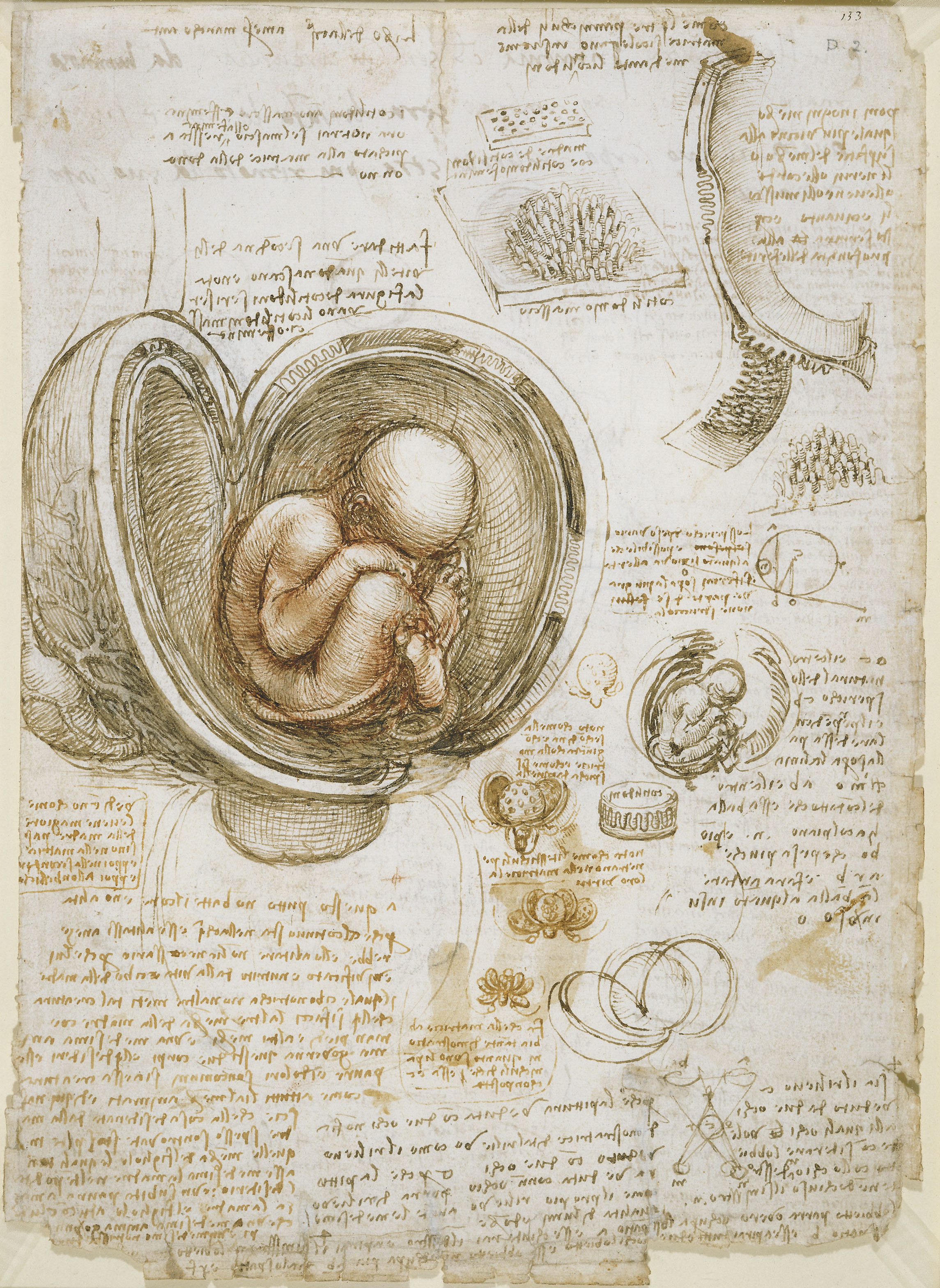
Foetus in the womb (c. 1510) Author Leonardo da Vinci (PDM) Public Domain
Leonardo made tremendous discoveries. He was the first to accurately depict the human spine. His notes described detailed documentation of liver cirrhosis. At the time the heart was believed to be a two-chamber system but Leonardo was the first to state in his notes that the heart was a four-chambered system. Not only this, he also correctly determined the pumping nature of the heart stating that two atria or “filling chambers” contract together while two ventricles or “pumping chambers” are relaxing. Leonardo noticed how spears that have been plunged into the hearts of pigs moved as the heart pumped, and using this information with his sketches he correctly determined the twisting nature of heart contractions. However, all these findings are overshadowed by Leonardo’s complex and integrated understanding of the hydrodynamic properties of the aortic valve.

The aortic valve (c.1512–13) Author Leonardo da Vinci (PDM) Public Domain
In 1968 two engineers B. J. Bellhouse and F. H. Bellhouse of Oxford University published a paper in nature discussing fluid dynamics of the aortic valve. The elementary way to describe valve function is that under the pressure of flowing blood the valves open and once the pressure is gone the backflow of blood closes the valve. However, it is a tad more complex than that. If those were the true dynamics of the heart the left ventricle would have a significant amount of blood back flowing into it before the valves could shut. Rather, little to no backflow is observed. This paper titled “Mechanism of Closure of the Aortic Valve” explored the true fluid dynamics of the aortic valve suggesting that the valve creates a turbulent flow of blood that results in quick and more efficient valve closure. This paper only had one citation and it was Leonardo Da Vinci (Note: The cite is actually K. D. Keele 1952 “Leonardo da Vinci on Movement of the Heart and Blood” which is simply a review of Leonardo’s circulatory work. However, the concept still stands, all the original work in this book was Leonardo’s). Leonardo made this realization approximately 450 years before these engineers by building a glass model of an ox heart’s sinus of Valsalva and pumping water with grass seeds through it to observe the flow. In this beautifully elegant experiment, Leonardo observed these vortexes and correctly hypothesized there importance.
Now if you’re anything like me, you’re wondering why Dr. Payne mentioned nothing about Leonardo da Vinci in our history of medicine lecture. Leonardo had completed a majority of his anatomical work by 1513 and Andreas Vesalius the anatomy artist discussed in class was born in 1514 (It has even been suggested that Andreas Vesalius had access to some of Leonardo’s early anatomical work). The answer to this question is simple. Leonardo did not publish any of his work. He only kept unorganized notes of the many experiments he performed. To me, this is what made Leonardo special. He did not research for recognition, fame, or praise. He asked a question simply because he had an insatiable curiosity for the discovering the world. Leonardo maintained what I would describe as a “Child’s mind” far into his adult years. He chose to ask “Why” and never made learning a chore, but rather kept it as the adventure it was meant to be. He is, to me, the truest symbol of innovation and wonder that has existed in the brief history of this earth. I think we can learn from Leonardo and hopefully, you feel inspired to explore your world and never stop asking “Why”.
If you have not gotten your Leonardo da Vinci itch out yet, here is a long documentary about his history in anatomy.
]]>Human aging is a complex phenomenon that results from environmental, genetic, and epigenetic events in various cell types and tissues. A prominent feature of aging tissues is inflammation. This process is referred to as inflammaging and is a high significant risk factor for both the diseased state and death of elderly people. However, the etiology of inflammaging and its causal role in unfavorable health outcomes is yet to be determined.
Why explore the relationship between aging and inflammation?
Different phenotypes of aging are associated with and can be predicted by elevated levels of biomarkers such interleukin-6 (IL-6). This inflammatory biomarker is present in states of mild inflammation and can be used to foretell many age-related phenotypes such as alterations in body composition, energy production and utilization, metabolic homeostasis, and neuronal health. Identifying pathways that control age related inflammation may shed light onto whether treatments that modulate inflammation could benefit elderly.
Types of inflammation
- Acute inflammation. The acute inflammatory response is vigorous within the first few hours or days but then gradually declines. Acute inflammation is beneficial as it induces an immune response and facilitates the healing, replacement, and adaptation of various tissues to conditions that may be detrimental such as tissue damage or intrusion of pathogenic microorganisms.
- Chronic inflammation. Chronic inflammation has many features of acute inflammation, but it is not related to the duration of the inflammatory response. A persuasive feature of aging is chronic inflammation, which often results in tissue deterioration.
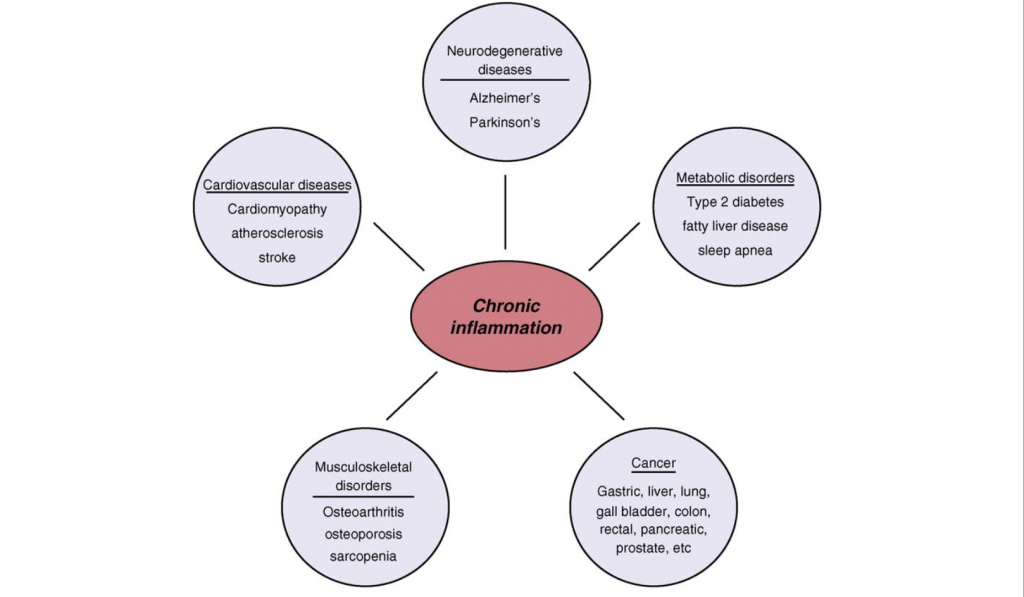
Figure 1.0. Chronic Inflammation associated with age related diseases. Chronic inflammation lies at the root of heart disease, cancer, osteoporosis, Alzheimer`s disease, diabetes and various other age-related diseases. Retrieved April 9, 2018. Source: Inflammatory networks during cellular senescence: causes and consequences. Trends in Molecular Medicine. 2010.
Stimulators of inflammation and the connection to aging
- The accumulation of cellular debris. As we get older, our body`s ability to eliminate cellular debris decreases significantly. This natural process continues as we age and cellular debris often builds up. These molecules are then processed by a network of sensors, such as the Nlrp3 inflammasome. The Nlrp3 inflammasome network labels the cellular debris molecules as “dangerous” and initiates an immune reaction that what will facilitate physiological repair. However, as cellular debris and damage increases, the responses can become chronic and hence maladaptive.
- Gut or oral microbiota. Harmful products produced by the human body, such as oral or gut microbiota, may spill over into nearby tissue and circulation. As we age, the liver’s ability to isolate these microbes and their product significantly decreases and it may result in chronic inflammation.
- Cell senescence. Senescent cells are associated as being the driving force behind aging and age-associated pathologies through their secretory phenotype. Mechanistically, senescent cells secrete numerous pro-inflammatory cytokines that modify the environments of tissues and the functions of neighboring cells. As we age, senescent cells accumulate and become abundant at the locations of various age-related pathologies. This aspect was demonstrated by a study that found the elimination of senescent cells in prematurely aged mice prevented many several age-related pathologies.
- The natural process of ageing likely causes changes in the immune system. Previous studies have found that the responses of adaptive immunity decrease as we age, whereas innate immunity experiences less drastic changes that may result in hyperactivity.
Cytokines
Cytokines are immunomodeling agents and are important features of chronic inflammation. Cytokines act as signaling molecules that aid cell to cell communication in immune responses and stimulate the movement of cells towards sites of inflammation, infection and trauma. Generally, cytokines bind to cell surface receptors and this commences an intracellular signaling cascades that ultimately activates transcription. Among this process, transcriptional factors, such as the proteins NF-κB (nuclear factor kappa-light-chain- enhancer of activated B cells) and STAT-3 (signal transducer and activator of transcription) regulate inflammation across several diseases and tissues.
NF- κB regulates genes that code for inflammatory cytokines as a previous study has found that NF-κB regulates the majority of genes that comprise the SASP. Additionally, NF-κB is a caustic factor in several aging phenotypes, particularly in the skin, spine, and brain Additionally, the activation of STAT-3 is emerging as a crucial mediator in inflammation as most proinflammatory agents have been shown to activate this factor. In regard to age related pathologies, IL-6 is a significant cytokine as it possesses a strong chronic inflammatory component. IL-6 mediates its effects through the activation of the STAT-3 pathway and is used a trademark of chronic morbidity. Further examples of cytokines that stimulate an immune reaction and elevate across multiple age-related diseases include IL-1β and tumor necrosis factor-α (TNF- α).
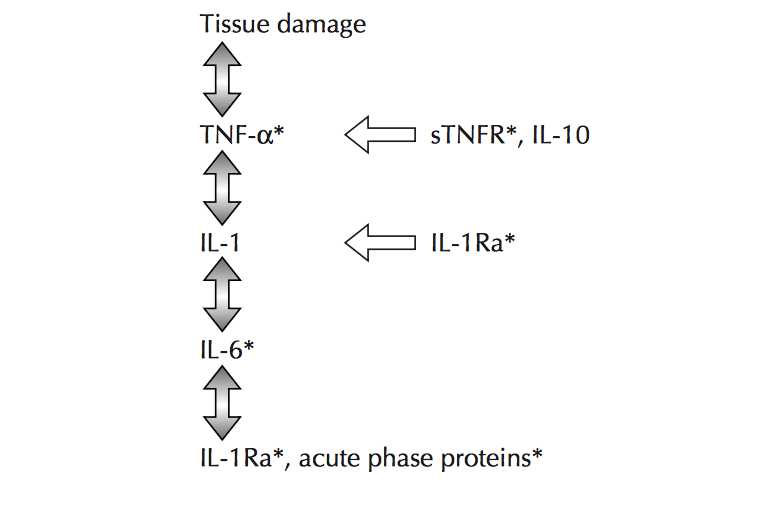
Figure 2.0. The inflammatory cascade including early mediators/proinflammatory cytokines TNF- α, IL-1β and distal mediators IL-6. Retrieved April 4, 2018. Source: Aging and inflammatory cytokines. Current Opinion in Hematology. 2001.
Interventions to prevent/control chronic disease
Medical advances that not only understand the characteristics of chronic inflammation, but also provide mechanisms to prevent and suppress chronic inflammation, have high potential for handling and/or preventing varying age-related pathologies. Anti-inflammatory interventions, such as the use of low dose aspirin, are already popular in clinical use. Further, considering the close relationship between adipose tissue and inflammatory response, interventions aimed at controlling obesity may be helpful as obesity provides a reservoir of inflammatory reactions. Similarly, a previous study has suggested that increasing levels of exercise may lower morbidity by lowering chronic inflammation.
Recent studies are also manipulating cell senescence. In particular, one study has found that eliminating senescent cells hold promise for lessening chronic inflammation as the elimination of these cells in a mouse model improved several age-related pathologies.
Conclusion
Inflammaging describes the low-grade chronic inflammation in aging and is a high significant risk factor for both the disease state and death of elderly people, however, preventative and curative measures may be taken. While acute inflammation may be beneficial as a fundamental immune response to damaging intracellular situations, chronic inflammation is often low grade and perpetual and results in tissue damage. Additionally, many stimuli activate inflammaging and there is the coming together of basic mechanisms and pathways such as activation of NF-κB and Nlrp3 inflammasome. However, little information is known about how these mechanisms/pathways are common amidst different organs and cell types as well as among age-related diseases. Forthcoming studies aimed at reducing chronic inflammation by senescent cells hold great promise for future treatment modules.
]]>
You may be thinking, “I took science 8, I know there are only two types of diabetes.” So you clicked the link hoping to find a place to comment “there’s a typo in your title” but actually, there’s no typo. Yes, you read that right, there is no typo in my title. What on earth is type 3 diabetes, you ask? Great question! Keep reading to find out.
What is Diabetes
Just so we’re all on the same page, let’s start with a basic overview of what on earth diabetes is. You may have a broad understanding that it has something to do with insulin and pricking your finger (eek blood), which is true, but I’d like you to know a little more than that. When you eat, you take in a bunch of glucose. That’s a good thing, your body needs glucose- and lots of it- because your body uses it to make energy. But you don’t want to make a bunch of energy right away and then be super tired in like an hour. So your body releases insulin, which tells your cells to take glucose and store it. In type 1 diabetes, the cells that make insulin are destroyed. Without insulin, your body has no way of telling your cells to store glucose. If you don’t store glucose, you can’t make energy later, and if you can’t make energy cells start to die. So insulin is very important. While type 2 diabetes has a similar set of consequences, there isn’t really a solid answer for what causes it. Obesity is definitely linked to type 2 diabetes, but so is having no fat on your body. So let’s just say both type 1 and type 2 diabetes result in a decreased intake of glucose. Okay, now that we have a solid foundation for what diabetes is, let’s move on.
Figure 1. A common picture that comes to mind when thinking about diabetes: the dreaded finger poke. Image credits: Reversing Your Diabetes Today (author). Creative Commons CC0 1.0 Universal Public Domain Dedication license.
What is This Type 3 Diabetes
Ladies and gentlemen, boys and girls, are you ready for the grand reveal of type 3 diabetes? Drum roll, please… type 3 diabetes is Alzheimer’s disease! Whaaaat? I know, I didn’t see it coming either. Worry not, in case you’re not overly familiar with what Alzheimer’s disease (AD) is, I will give you some background information. Quickly, AD is a neurodegenerative disorder (say that five times fast) that’s characterized by a decline in cognitive function. It usually begins with memory problems, then people will get confused, agitated, and have behavioral troubles, and then it continues to gets progressively worse. How is that in any way similar to people that would essentially starve- even though they’re eating- if they didn’t take insulin? Another great question insightful reader! Let’s delve into the answer to that.
Type 3 Diabetes
The thing this, there’s a lot of markers for Alzheimer’s disease in the brain- things like loss of cells and plaque build-ups- and it’s hard to link all these things to one causational factor. What’s gaining a lot of popularity as the cause for all these markers though, is a decrease in the use of glucose. More specifically, this decrease is thought to be because of some impairment of insulin. Well, that sounds a little familiar, doesn’t it? People that have AD show both a decrease in insulin production and a resistance in insulin receptors. What that means, is that only a little bit of insulin is made. And when this little bit of insulin tries to land on a cell and kindly ask it to take in some glucose, the cell doesn’t recognize it. So it pretty much shuts the door on insulin’s metaphorical face. Well did it ever occur to anyone that maybe diabetes causes Alzheimer’s? Another great question, my gosh, you are an intelligent reader! The answer is yes; it definitely has occurred to people that diabetes may just be a risk factor for AD.
Type 1 and 2 Diabetes and Alzheimer’s Disease
We know that Alzheimer’s disease, like diabetes, involves some wonky insulin, so it’s super logical to think that diabetes causes AD. Also, both obesity and diabetes are risk factors for Alzheimer’s disease. So that hypothesis is looking pretty good right about now. Are you waiting for the other shoe to drop? Here is it: while you can definitely get AD if you have diabetes, you can also get wonky insulin and AD without either type one or type two diabetes. And, in a nutshell, there are characteristics of AD that overlap with characteristics of type one and type two diabetes, but AD doesn’t mimic either of these, so it’s not a by-product of them.
What Does This Mean
I’m a total nerd, so this is enough to excite me. But I understand that not everyone is like me, you may need some big picture ideas to get excited about this. I respect that, and hopefully, I can provide that for you. Alzheimer’s is a horrible disease. Talking to a loved one that doesn’t remember you and watching them no longer be able to do things for themselves is heartbreaking. But if we can treat AD with diabetes medication (which has been shown to work) then maybe we could prevent, or at least prolong the progression, of this! I’m talking cure people!!* I know some people think cognitive disorders are just a by-product of aging, but this research shows that it’s so much more than that. And more so than that, it shows that leading a healthy lifestyle may prevent cognitive disorders! So there’s added incentive for people to get healthy and stay active. If that doesn’t blow your mind, I’m sorry I’m at a loss. Nonetheless, thank you for reading my blog post and stay healthy people!
*Disclaimer: it’s much too early in the research process to assume that diabetes medication is a cure for AD, but it is certainly a possibility!
]]>