The AllTrials Campaign
Incomplete or unreported clinical trials distort the evidence base and erode public trust. The global AllTrials campaign is making sure every trial – past and present – is registered and reported.
Support our workIn 2013, we estimated over half of all clinical trial results were never reported. Our response was the global AllTrials campaign — an international initiative of Ben Goldacre, the BMJ, the Centre for Evidence-based Medicine, the Cochrane Collaboration, the James Lind Initiative, PLOS and Sense about Science. Our goal: for every clinical trial to be registered and their full methods and results reported – even if they are negative.
Why registration and reporting of clinical trials matters
When the results of clinical trials are not reported, the public trust in science is damaged — a loss we cannot afford in an era where mis- and disinformation run rampant.
Withheld clinical trial results threaten the public trust in science, inhibit potentially life-saving research, waste resources, prevent fully informed treatment decisions and pose an unacceptable risk to human life.
“We know that trials with less flattering results…are more likely to go missing in action than trials with positive results. And so, as a consequence, we know that the half of the results we’re seeing are biased.”
Professor Ben Goldacre, co-founder of the AllTrials campaign
When results are cherry-picked, the evidence we have access to is distorted. We’re only able to understand the data through the lens those who handle it want us to, and this suggests that we rarely ever see the trial results that are negative. Regulatory bodies, clinicians and doctors are then unable to make effective, informed decisions about important healthcare interventions.
When members of the public know their healthcare providers are only partially informed, it creates more uneasiness in a time that’s already stressful. Many clinical trials are also publicly funded, and researcher accountability provides us answers as to how our money is spent.
Further, volunteers take part in these trials without knowing anything about the efficacy or safety of the intervention, which poses an inherent risk. Given this risk, ensuring results are reported and volunteers are aware of the intervention’s impacts is a basic moral duty as well.
Everyone feels the impact of this malpractice. Clinicians and doctors can’t fully understand the interventions they’re tasked with evaluating, patients face additional uncertainty, and volunteer subjects are left in the dark, leaving us – the group whose funding allows these trials to take place – to bear the brunt of it.
We are witnessing never-before-seen advances in healthcare, and these advances have the potential to save lives across the world. But when results are withheld from the public, scepticism arises, treatments are misjudged and our trust in scientific research is damaged.
Public and professional demand for change
While the issue with unreported medical trials was already well-recognised by medical bodies and the pharmaceutical industry, the mission needed a catalyst. AllTrials has become the public campaign to channel collective global concerns and demand change.
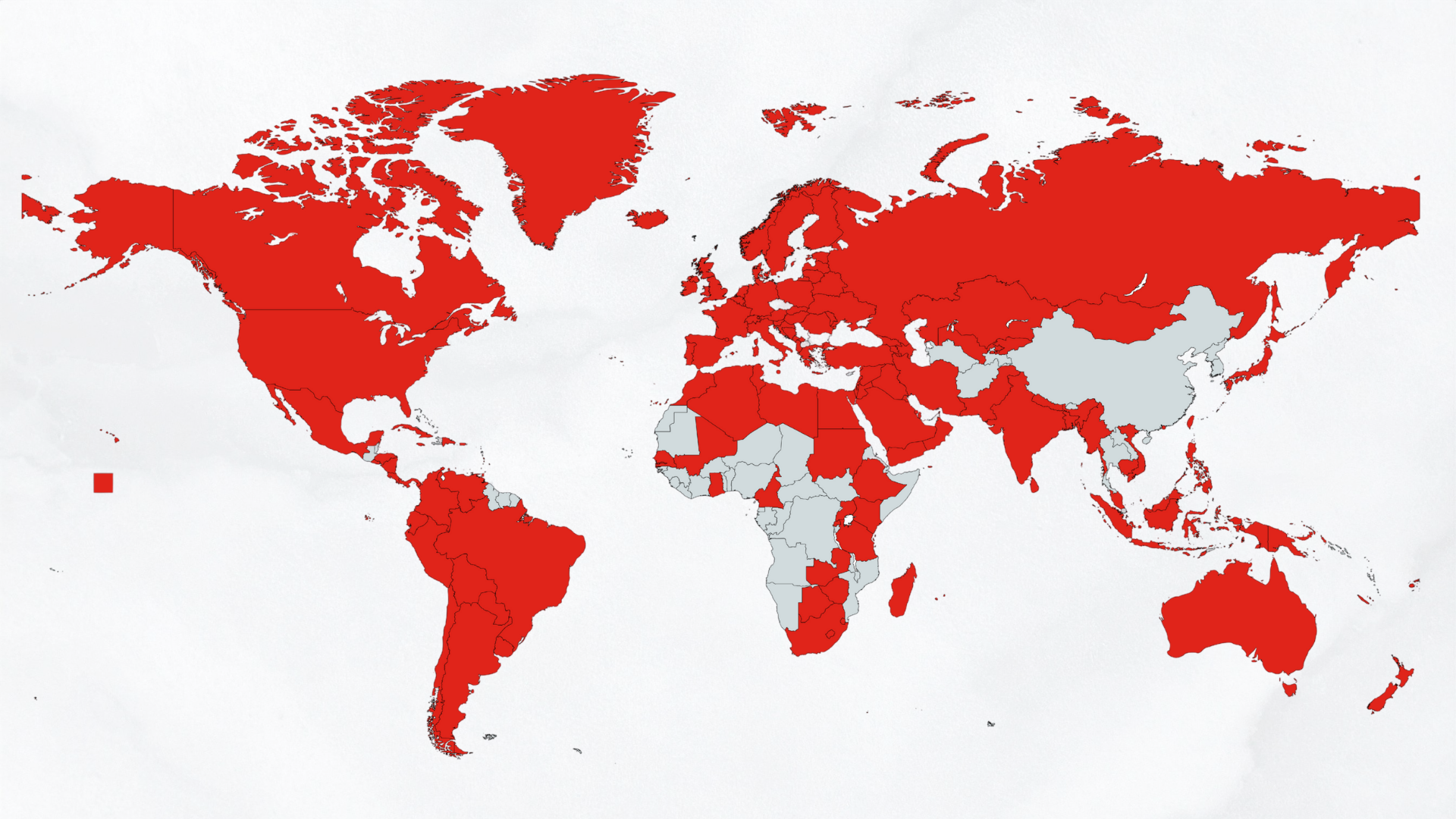
We must make sure governments across the world know that change is non-negotiable. The overwhelming support for the AllTrials initiative shows the global interest in research transparency and pressures policymakers all over the world to reevaluate the standards by which clinical researchers must adhere.
“What the campaign did, really, was give people who are already talking about this and doing something towards it the support—the courage even—to keep going”
Sile Lane, a founder of the AllTrials campaign
Stronger clinical trials regulations
Since the inception of the AllTrials initiative, tangible, enforceable amendments to research regulations have been achieved. The campaign has also received recognition from the BBC,
which shared its successes as a rare piece of good news for research, and it is cited as a movement that inspired worldwide action.
United Kingdom
New regulations developed by the Medicines and Healthcare Products Regulatory Agency (MHRA) and the Healthcare Research Authority (HRA) will come into force on 28 April 2026. By law, all clinical trials in the UK will be required to:
-
- When trials are registered on a public registry, we can easily identify trials performed in the UK, and sponsors can easily disseminate their results.
- The HRA is partnered with a main public registry, ISRCTN, which streamlines trial registration and application, making the process seamless for researchers as well.
- Registration with the EU’s Clinical Trials Information System (CTIS) will be inadequate, as it does not allow us to identify trials taking place in the UK.
-
- Sponsors must publish summaries of their protocol and results through the registry the trial is published with. It is highly encouraged for them to do so in plain language so we can all understand what they mean.
- Being clicks away from timely, comprehensive trial summaries means clinicians, doctors and we, the public, have access to science-based answers that can save lives (and taxpayers’ money).
- To ensure these summaries are clear, the qualifying registries ISRCTN and ClinicalTrials.gov are both available in Be Part of Research, which further breaks down trial summaries and their implications
-
- Beyond publishing results publicly, sponsors are obligated to actively offer complete summaries to volunteer subjects.
- They must provide these summaries to each volunteer in plain language, and the results shared must reflect the trial as a whole instead of on an individualised basis.
- Without the volunteers who subject themselves to unknown risks in the name of advancing healthcare for others, the introduction of tested, safe medical interventions would be stalled significantly. Answers are the least we can offer them.[
-
We are surely closer than we were. Adherence will be monitored by the HRA and enforced by the MHRA, with punishments for offences including:
- Fines
- Imprisonment
- Denial of future trial approval if a sponsor has not complied with regulations
The amendment also acknowledges the transitional period as the amendment is introduced and explains how enforcements will be arranged during this time.
European Union
The European Union simplified the registration and reporting of clinical trials. Since 31 January 2023, all initial clinical trial applications in the EU must be submitted via the CTIS, making it the single-entry point for the submission, assessment and dissemination of clinical trial data collected in the EU.
USA
In the USA, the Food and Drug Administration Amendments Act of 2007 required sponsors running clinical trials of drugs and devices via funding from the National Institutes of Health (NIH) must register them on ClinicalTrials.gov within 21 days of enrolling the first volunteer, but this law was never effectively enforced. However, in 2023 the Government Accountability Office published a report which found that between July and November 2022, the agency brought 235 researchers into compliance with registration and reporting requirements
Global
At the 75th World Medical Association General Assembly in October 2024, we witnessed a groundbreaking update to the Declaration of Helsinki, which regulates medical practices worldwide. The amendment states that the dissemination of results is an ethical obligation, negative and inconclusive results should be published and all affiliations (e.g., funding sources, conflicts of interest) should be disclosed. Any research which does not adhere to these principles should not be accepted for publication.
All of these regulations have an impact even in jurisdictions where there are no strong laws because many big clinical trials are international. If a study has one clinic in a country with regulations – and the UK has traditionally been popular for drugs trials because of the National Health Service (NHS) infrastructure – then that applies to results from everywhere
Next steps for clinical transparency
We campaign for transparency of evidence in all areas: AllTrials is perhaps the most tangible example of the social harms that result when knowledge and data are not openly shared.
Over the past decade, there has been significant progress in clinical trial registration and reporting. We have achieved recognition of and respect for the volunteers, whose participation these trials depend completely, and we allowed clinicians, regulatory bodies and doctors access to more tools to help them better care for their communities. As the potential subjects of these interventions, we’re entitled to receive care based on fully informed decisions.
But until 100% of clinical trials are registered and 100% of their results reported, there is still work to do. We invite you to sign up for updates from us – we will keep you informed of progress and ways you can help in your area.
AllTrials partners and supporters
+ Click to view the list of all supporting organisations
Academy of Medical Royal Colleges
ACCYPN: Australian College of Children & Young People’s Nurses
ACHSE e.V. (German National Alliance for Chronic Rare Diseases)
Addison’s Disease Self Help Group
Adelaide Health Technology Assessment
Advanced Emergency Nursing Journal
AEP (Spanish Evidence-based Paediatrics)
AIDS Coalition to Unleash Power Paris
AIDS Treatment Activists Coalition
All Ireland Institute of Hospice and Palliative Care
Alzheimer’s Australia Dementia Research Foundation
American Academy of Emergency Medicine
American Academy of Emergency Nurse Practitioners
American Academy of Family Physicians
American Autoimmune Related Diseases Association
American Behcet’s Disease Association
American Board of Sport Psychology
American College of Physicians
American Institute for Technology and Science Education
American Medical Student Association
American Medical Women’s Association
American Psychiatric Association
amfAR The Foundation for AIDS Research
Anxiety and Depression Association of America
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesselschaften
Arthritis and Musculoskeletal Alliance
Asociación Española de Pediatría de Atención Primaria
Association for Pelvic Organ Prolapse Support
Association for the Advancement of Experimental and Applied Hypnosis
Association of British Clinical Diabetologists
Association of Liberal Democrat Engineers and Scientists
Association of Medical Research Charities
Associazione Alessandro Liberati – Network Italiano Cochrane
Associazione Salute Attiva Onlus
Australasian College for Emergency Medicine
Australasian College of Nutritional and Environmental Medicine
Australasian Medical Writers Association
Australian Medical Students’ Association
Australian Pain Management Association
Avon Primary Care Research Collaborative
Balance – Familienplanungszentrum
Belgian Centre for Evidence Based Medicine (cebam)
Belgian Health Care Knowledge Centre (KCE)
BEUC The European Consumer Organisation
BioNorte – Basque Health Sciences Society
Birmingham Skeptics in the Pub
Boston University School of Public Health
Bristol and Avon Chinese Women’s Group
British Association for Counselling and Psychotherapy
British Association of Dermatologists
British Council for Prevention of Blindness
British HIV Association (BHIVA)
British Institute of Radiology
British Obesity Surgery Patients
British Paediatric Allergy, Immunology and Infectious Diseases Group
British Pharmaceutical Students’ Association
British Pharmacological Society
British Society for Gene and Cell Therapy
British Society for Immunology
British Society for Sexual Health and HIV
British Society for the Study of Vulval Diseases
British Society of Periodontology
British Veterinary Association
Bundesverband Prostatakrebs Selbsthilfe e. V.
Bury Knowle Patient Participation Group
Canadian Agency for Drugs and Technologies in Health
Canadian Cancer Research Alliance
Cancer Prevention and Treatment Fund
Cardiff University Systematic Review Network (SysNet)
CEMBE (Centro de Estudos de Medicina Baseada na Evidência)
Center for Evidence-Based Physiotherapy
Center for Evidence-based Policy, Oregon Health & Science University
Center for Information and Study on Clinical Research Participation
Centre for Evidence Based Policy
Centre for Evidence-based Veterinary Medicine
Centre for Reviews and Dissemination, University of York
Centre for Statistics in Medicine
Centre for Sustainable Healthcare
Centre of Evidence Based Dermatology
Centro Nacional Coordinador de Ensayos Clínicos (CENCEC)
Charité – Universitätsmedizin Berlin
Children’s Liver Disease Foundation
Chronic Granulomatous Disorder Society
Chronic Pain Research Alliance
CILIP: the Chartered Institute of Library and Information Professionals
Clinical Data Interchange Standards Consortium (CDISC)
Clinical Trial Service Unit & Epidemiological Studies Unit, University of Oxford
Cochrane Eyes and Vision Group
College of Mental Health Pharmacists
College of Psychiatric and Neurologic Pharmacists
COMCEPT – The Portuguese Skeptics Community
Committee On Publication Ethics
Comsumers United for Evidence-based Healthcare
Connecticut Veterinary Medical Foundation
Consumers Health Forum of Australia
Contact Help Advice Information Network
Critical Appraisal Skills Programme International Network
Critical Appraisal Skills Programme Mexico
Critical Appraisal Skills Programme Spain
Critical Appraisal Skills Programme UK
Dalhousie AllTrials Student Society
Dartmouth Institute for Health Policy and Clinical Practice
Daughters of Charity, Province of St. Louise
Deutsche Dupuytren-Gesellschaft
Deutsche Gesellschaft für Neurologie
Deutsche Gesellschaft für Soziale Psychiatrie
Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung
Deutscher Psoriasis Bund e.V. (DPB)
Dr. Susan Love Research Foundation
Drug and Therapeutics Bulletin
Drug Commission of the German Medical Association
Drug Information Center of National University of Colombia (CIMUN)
Dutch Association of Pharmaceutical Medicine
Estonian Medical Students’ Association
Ethics Committee of Provincies of Verona and Rovigo
European Academy of Paediatrics
European Association of Hospital Pharmacists
European Association of Science Editors
European Cancer Patient Coalition
European Central Council of Homeopaths
European Clinical Research Infrastructure Network
European Continuing Medical Education Forum
European Federation of Clinical Chemistry and Laboratory Medicine
European Federation of the Associations of Dietitians
European Journal of Hospital Pharmacy
European Prostate Cancer Coalition (Europa UOMO)
European Public Health Alliance
European Public Health Association (EUPHA)
European Society of Clinical Pharmacy
Evidence Synthesis and Modelling for Health Improvement
Evidence-Based Veterinary Medicine Association
Faculty of Pharmaceutical Medicine
Faculty of Sexual and Reproductive Healthcare
Failed Implant Device Alliance
Foreningen Skepsis (The Norwegian Skeptics Society)
Foundation for Integrative AIDS Research
Fundació Institut Català de Farmacologia (FICF)
George & Fay Yee Centre for Healthcare Innovation
German Association of Hospital Pharmacists (ADKA)
German Clinical Trials Register
German Institute for Quality and Efficiency in Health Care (IQWiG)
German League of People Against Rheumatism
German Medical Association of Applied Kinesiology DÄGAK
German Network for Evidence Based Medicine (ebm)
German Public Health Association
German Society for Health Technology Assessment (HTA.de)
German Society for Wound Healing and Wound Treatment
Gesellschaft zur wissenschatlichen Untersuchung von Parawissenschaften e.V.
Global Healthcare Information Network
Groupe de Recherche et d’Action pour la Santé
Guild of Healthcare Pharmacists
Haematology Clinical Trials Unit
Health Action International Europe
Health Action International Global
Health and Care Research Wales Support Centre
Health Faculty at Universidad del Valle
Healthcare Information For All
Histologie Zytologie Laboratorium Nussdorf
HLF Hørselshemmedes Landsforbund
Hospital Pediatrico de Sinaloa (HSP)
Hospital Pharmacists Association Ireland
Hungarian League Against Cancer
Hypermobility Syndromes Association
Independent Cancer Patients’ Voice
Initiative for Medicines, Access & Knowledge
Institute for Evidence Based Medicine, College of Medicine, Korea University
Institute of Physics and Engineering in Medicine
Integrative Psychological Services of New York City
Intensive Care National Audit and Research Centre
International Academy of Nursing Editors (INANE)
International Alliance of Patients’ Organizations
International Brain Tumour Alliance
International Coalition for treatment preparedness in Eastern Europe and Central Asia
International Federation of Anthroposophical Medical Associations
International Federation of Medical Students’ Associations
International HIV/AIDS Alliance
International Institute for Advanced Studies of Psychotherapy and Applied Mental Health
International Journal of Gynecology and Obstetrics
International Physicians for the Prevention of Nuclear War
International Rectal Microbicide Advocates
International Society for Evidence Based Health Care
International Society of Drug Bulletins
International Treatment Preparedness Coalition (ITPC)
International Union of Basic & Clinical Pharmacology (IUPHAR)
IPF Support Group of Montgomery
Iranian Food & Drug Organisation
IRCCS – Mario Negri Institute for Pharmacological Research
Irish Platform for Patients’ Organisations, Science and Industry (IPPOSI)
Italian Association of Neuroepidemiology
Italian Federation of Volunteer-based Cancer Organisations (FAVO)
James Whale Fund for Kidney Cancer
Joe Walsh Memorial Pulmonary Fibrosis Support Group
Journal of Cognitive and Behavioural Psychotherapies
Journal of Kathmandu Medical College
June Hancock Mesothelioma Research Fund
Laarkarin Sosiaalinen Vatsuu – Physicians for Social Responsibility
Landsforeningen for Hjerte- og Lungesyke
Landsforeningen uventet barnedød
LILA Onlus – Lega Italiana per la Lotta contro l’Aids (Italian League for Fighting Aids)
Liverpool School of Tropical Medicine
London School of Hygiene & Tropical Medicine
Lung Cancer Research Foundation
Más Ciencia por México (More Science for Mexico)
Medical Sciences Division, University of Oxford
Mercy Investment Services, Inc
Mission Arogya Health and Information Technology Research Foundation
Mobility and Support Information Service
Motor Neurone Disease Association
Multippel Sklerose forbundet i Norge
Muscular Dystrophy Support Group
Nasjonalforeningen for folkehelsen
National Ankylosing Spondylitis Society
National Association of Deafened People
National Breast Cancer Coalition
National Collaborating Centre for Mental Health
National Committee for Research Ethics in Norway
National Comprehensive Cancer Network®
National Health and Medical Research Council of Australia
National Institute for Health and Care Excellence (NICE)
National Ovarian Cancer Coalition
National Partnership for Women & Families
National Rheumatoid Arthritis Society
National Union of Scientific Medical Information
Netherlands Epidemiological Society
New Zealand Medical Association
NHS Research & Development Forum
NHS Vale of York Clinical Commissioning Group
NISCHR Clinical Research Centre
Norges Astma- og Allergiforbund
Norges Myalgisk Encefalopati Forening
Norske Kvinners Sanitetsforening
North Wales Organisation for Randomised Trials in Healthcare
North Yorkshire Humanist Group
Northern California Regional Organization of Child and Adolescent Psychiatry
Norwegian Knowledge Centre for Health Services (Kunnskapssenteret)
Nottingham Clinical Trials Unit
Organisation for Anti-Convulsant Syndromes
Otago Medical Research Foundation
Otago University Medical Students Association
Ovarian Cancer Alliance of San Diego
Ovarian Cancer Research Fund Alliance
Oxford Clinical Trials Research Unit
Oxford University Hospitals NHS Trust
Pharmaceutical Journal (PJ Online)
Plymouth University Peninsula Clinical Trials Unit
Plymouth University Peninsula Schools of Medicine & Dentistry
Positive People Armenian Network
Pragmatic Clinical Trials Unit
Primary Care Respiratory Society UK
Prostate Health Education Network
Québec Medical Association (QMA)
Reconstructive Surgery Trials Network
RELIS: Regional medicines information & pharmacovigilance centre
Research Design and Conduct Service
Royal Australian and New Zealand College of Psychiatrists
Royal College of General Practitioners
Royal College of Obstetricians and Gynaecologists
Royal College of Paediatrics and Child Health
Royal College of Physicians of Edinburgh
Royal College of Psychiatrists
Royal National Institute of Blind People
Russian Society for Evidence Based Medicine
Scientists for Global Responsibility
Scottish Intercollegiate Guidelines Network
Society for the Improvement of Science
Society for Women’s Health Research (SWHR)
South African Medical Research Council
Southampton Clinical Trials Unit
Spanish Society of Medical Radiology
Swedish College of General Practice (SFAM)
Swiss Multiple Sclerosis Society
Syncope Trust and Reflex anoxic Seizures
Tatarstan Medical Students’ Association
Test-Achats, Belgian Consumer Organisation
The American College of Chest Physicians
The American College of Obstetricians and Gynecologists
The Association for Clinical Biochemistry
The Association of Democratic Pharmacists (The VdPP)
The Association of Research Ethics Committees
The British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS)
The British Society for Rheumatology
The Centre for Intervention Science in Maternal and Child Health
The Chartered Society of Physiotherapy
The Coalition Against Pediatric Pain
The Cochrane Hepato-Biliary Group (The CHBG)
The Dutch Society for Rheumatology
The Faculty of Intensive Care Medicine
The Guidelines International Network G-I-N
The Hepatitis C Mentor & Support Group
The Institute of Clinical Research
The Lymphoedema Support Network
The Nursing Midwifery and Allied Health Professions Research Unit
The Oxalosis & Hyperoxaluria Foundation
The Pernicious Anaemia Society
The Royal College of Ophthalmologists
The Royal College of Surgeons of England
The Swiss Skeptics (Skeptiker Schwiez)
The Welsh Intensive Care Society
The Women’s Research Initiative on HIV/AIDS
Transform Drug Policy Foundation
Transparency International Deutschland e.V.
Turkish Clinical Research Association
UK Clinical Pharmacy Association
UK Cystic Fibrosis Gene Therapy Consortium
UK Dermatology Clinical Trials Network
UK Translational Research Network in Dermatology
UKCRC Registered Clinical Trials Unit Network
United European Gastroenterology
Universities Allied for Essential Medicines
University Hospitals Coventry and Warwickshire NHS Trust
University of Bradford School of Pharmacy
University of Lisbon Faculty of Medicine
University of Oxford Diabetes Trials Unit
Verein demokratischer Pharmazeutinnen und Pharmazeuten
Western Australian Clinical Oncology Group
Wilson’s Disease Support Group UK
World Association of Medical Editors
World Confederation for Physical Therapy
YWCA Princeton Breast Cancer Resource Center
ZonMw, the Netherlands Organisation for Health Research and Development


